A Guide to Medication Packaging Systems for Patient Safety and Clinic Efficiency
Discover how medication packaging systems safeguard patients, ensure TGA compliance, and boost efficiency in chronic care.

Effective medication packaging systems are the foundation of patient safety, regulatory compliance, and operational workflow for any clinic or telehealth service. The primary conclusion is that these systems are not merely containers but critical infrastructure that ensures the right dose reaches the right patient securely, protects therapeutic integrity, and mitigates significant clinical and business risks. They are the first line of defence in a modern healthcare delivery model.
Understanding the Critical Role of Medication Packaging
In modern healthcare, particularly with the growth of telehealth and personalised medicine, medication packaging has evolved from a simple container to a sophisticated system integral to care delivery. An effective packaging system creates a secure, traceable, and error-proof journey for medication, from a pharmacy partner directly to the patient.
According to one regulatory affairs expert, "Medical device packaging should never be an afterthought. It's your first line of defense against regulatory nightmares, product failures, and recalls that destroy reputations overnight." This statement is even more critical for pharmaceuticals, where a packaging failure can have direct and severe consequences for patient health.
More Than Just a Container
While the fundamental purpose of any package is to protect its contents, its role in a clinical context is far more advanced. The right packaging directly influences patient outcomes and ensures the smooth operation of a clinic.
- Improving Dosage Accuracy: For patients on complex medication schedules, systems like multi-dose blister packs are transformative. They organize medications by day and time, which significantly reduces the risk of patients confusing medications or missing doses.
- Maintaining Medication Stability: Many modern pharmaceuticals are sensitive to environmental factors. Specialised packaging is engineered to protect them from light, moisture, and temperature fluctuations, ensuring the medicine remains effective from the pharmacy to the patient.
- Ensuring Regulatory Compliance: In Australia, the Therapeutic Goods Administration (TGA) enforces strict regulations, especially for Schedule 8 (S8) controlled substances. Compliant packaging, featuring elements like tamper-evident seals and child-resistant closures, is a legal requirement, not an option.
- Enhancing Fulfilment Efficiency: Automated medication packaging systems can integrate with pharmacy management software. For a telehealth provider, this translates to faster, more accurate prescription fulfilment and a reduction in manual errors that can delay dispatch.
As one clinical pharmacist noted, "These systems create an unbroken chain of accountability. From the moment a prescription is filled to the patient taking their medication, every step is secure and verifiable. This protects the patient, and it protects the prescriber."
The following table provides an overview of the most common systems.
Overview of Key Medication Packaging Systems
Packaging SystemPrimary Use CaseKey BenefitBlister PacksSingle-medication dispensing for retail or clinical trials.High product integrity and clear dose tracking.Unit-Dose PackagingHospital and institutional settings.Reduces medication errors and streamlines administration.Multi-Dose PacksPatients with complex, multi-drug regimens (e.g., aged care).Massively improves adherence and simplifies daily routines.Compliance PackagingChronic care management for patients at home.Empowers patient independence and reduces dosage confusion.Cold-Chain & VaultingTemperature-sensitive biologics and high-risk S8 substances.Guarantees medication stability and meets strict security mandates.
Each system is designed to address a specific clinical or logistical challenge, ensuring medication is not only delivered but delivered correctly and safely.
The Real-World Impact on Patient Adherence
One of the most significant benefits of a well-designed medication packaging system is its positive impact on patient adherence. Managing multiple prescriptions for a chronic condition can be overwhelming. Compliance packaging simplifies this process, empowering patients to adhere to their treatment plan without daily stress or confusion.
This is particularly crucial for treatments like medicinal cannabis, where consistent and precise dosing is key to achieving therapeutic outcomes. You can explore the nuances of these products in our article on Australia's medical cannabis landscape. Ultimately, the objective is to make adherence feel effortless for the patient, which leads to better health outcomes and a more reliable care model for the clinic.
Diving Into the Different Types of Medication Packaging
No single medication packaging solution fits all scenarios. These systems are specialised tools, each engineered for a specific function within a particular clinical environment. Understanding the distinctions between Dose Administration Aids (DAAs), unit-dose packs, and specialised vials is essential for selecting a system that enhances both patient safety and operational workflow.
This hierarchy diagram illustrates the key considerations behind the design and selection of these systems.
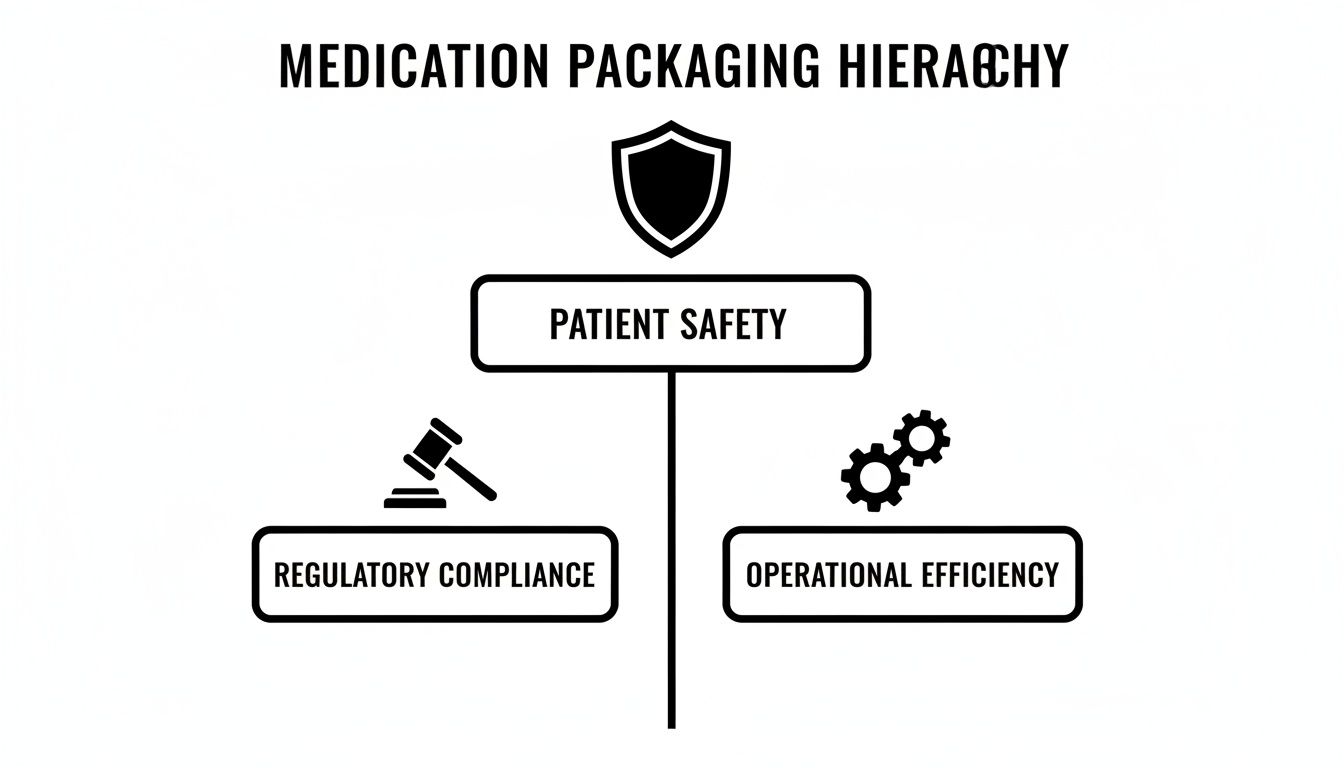
As shown, patient safety is the primary priority, supported by a foundation of regulatory compliance and operational efficiency.
Multi-Dose Blister Packs: The Daily Organiser
For patients managing multiple medications, multi-dose blister packs—a common type of Dose Administration Aid—function as a daily health organiser. These packs group various pills and capsules into sealed compartments, clearly labelled for specific times of day, such as morning, noon, or evening.
This system effectively pre-sorts a week's worth of medication into a simple format. This removes guesswork and reduces the risk of error in a patient's daily routine, which is vital for managing chronic conditions where consistency is paramount. The clear layout helps prevent common errors like missed doses or accidental double-dosing.
This approach is widely used in community pharmacies and aged care. A community pharmacist explains, "The goal is to make medication adherence effortless." Multi-dose packs achieve this by removing the cognitive burden of managing multiple pill bottles, giving patients the confidence to follow their treatment plan accurately.
Unit-Dose Packaging: Precision for Institutions
In contrast, unit-dose packaging is designed for high-volume, controlled environments like hospitals or large-scale clinical fulfilment centres. Each packet contains a single dose of one specific medication, individually wrapped and labelled with critical information such as the drug name, strength, lot number, and expiry date.
The primary function of unit-dose packaging is to minimise medication administration errors. In a busy hospital setting, a nurse can scan the barcode on a unit-dose package, verify it against the patient's electronic health record, and administer it, creating a closed-loop system that significantly enhances safety.
This system provides an exceptional level of traceability and control. From the moment the medication is packaged until it is administered, there is a clear, verifiable audit trail that supports institutional accountability and simplifies inventory management.
While not typically used in a home setting, its precision is non-negotiable in facilities where hundreds or thousands of doses are administered daily. The efficiency gained from this streamlined process makes it the gold standard in institutional pharmacy. To see this precision applied in a specialised context, you can learn about the zero-error fulfilment process for medicinal cannabis.
Specialised Vials and Containers: Handling Sensitive Medications
Not all medications can be placed in standard blister packs. Biologics, certain compounded formulations, and drugs sensitive to light require specialised packaging to maintain their stability and efficacy. This is where options like amber-tinted vials, refrigerated containers, and secure bottles are essential.
These containers are engineered to provide specific types of protection:
- Light Protection: Amber glass or opaque plastic vials are used to block UV rays that can degrade light-sensitive medications.
- Moisture Control: Airtight seals and desiccants are often included to protect drugs that are susceptible to damage from humidity.
- Tamper-Evidence: Secure caps and seals provide a clear visual indicator if the container has been opened, guaranteeing product integrity.
The choice of container is a clinical decision based on the chemical properties of the medication. The rationale is simple: without appropriate protective packaging, a drug can lose its therapeutic potency before it reaches the patient, undermining the entire treatment. This is particularly true for high-value or Schedule 8 drugs, where product integrity is non-negotiable.
Meeting TGA and Schedule 8 Compliance Requirements
For Schedule 8 (S8) controlled substances, advanced medication packaging is the bedrock of regulatory compliance. In Australia, the Therapeutic Goods Administration (TGA) has a comprehensive regulatory framework governing every aspect of pharmaceutical handling. Adherence to packaging standards is non-negotiable.
The packaging serves as physical evidence of a clinic's commitment to safety and accountability. This is not merely a box-ticking exercise; it is about building a robust system that protects patients, reduces prescriber liability, and ensures operations remain audit-ready.

Building a Validated Chain of Custody
A critical function of compliant medication packaging is to establish a validated chain of custody. This is an unbroken, auditable trail that tracks a medication from the pharmacy vault to the patient. For S8 medications, this is a paramount requirement.
This chain of custody is dependent on several key packaging features:
- Tamper-Evident Seals: These provide immediate, clear visual evidence if a package has been compromised. A broken seal acts as a critical alert for both the patient and the provider, preventing the use of a potentially unsafe substance.
- Child-Resistant Mechanisms: TGA regulations mandate child-resistant closures as a fundamental safety measure to prevent accidental ingestion, particularly for medications delivered to a patient's home.
- Accurate and Durable Labelling: Labels must contain specific information—patient name, drug, dose, expiry date—and remain fully legible throughout the product's lifecycle, resisting smudging or peeling.
These elements work in concert to create a secure physical barrier around the medication, which serves as evidence of due diligence during regulatory inspections.
The Role of Packaging in S8 Vaulting and Inventory Management
For clinics and telehealth platforms, managing S8 medications requires specialised infrastructure. TGA-compliant packaging is intrinsically linked to how these high-risk drugs are stored and tracked, especially within a segregated inventory system.
"For controlled substances like Schedule 8 medications, compliant packaging is the first and most critical control point," explains a pharmaceutical logistics specialist. "It's what allows for secure vaulting, segregated inventory, and a defensible audit trail, ultimately mitigating risk for both the patient and the prescriber."
Secure S8 vaulting relies on packaging that facilitates accurate tracking. When each unit is serialised and sealed, precise inventory counts and reconciliations are possible, making drug diversion extremely difficult to conceal.
This level of control is fundamental to TGA compliance. The demand for such secure solutions is growing, as evidenced by market data. Australian biopharmaceutical packaging market insights show the market was valued at USD 418.18 million and is projected to reach USD 921.83 million by 2033, a surge driven by investment in packaging for controlled medications.
Mitigating Risk and Ensuring Audit Readiness
Ultimately, TGA and S8 compliance is about risk mitigation. By implementing appropriate medication packaging systems, clinics and prescribers significantly reduce their liability. A robust packaging strategy is a proactive measure to prevent medication errors, drug diversion, and accidental harm.
In the event of a regulatory audit, the packaging itself becomes a critical piece of evidence. Proper labels, tamper-evident seals, and comprehensive documentation demonstrate adherence to protocol. For telehealth services, this is even more crucial, as the physical distance between prescriber and patient places greater emphasis on the integrity of the fulfilment process.
For a detailed breakdown of these processes, refer to our article on rapid Schedule 8 medication fulfilment for telehealth.
Integrating Packaging with Telehealth Fulfilment
Telehealth has fundamentally reshaped healthcare delivery, creating a critical need for medication packaging systems that integrate directly into digital workflows. This integration facilitates a seamless process from the prescriber's interface to the patient's home.
For modern clinics, this integrated system is essential for moving prescriptions with speed, accuracy, and complete traceability. The core of this integration lies in the connection between physical packaging and digital fulfilment technology.
At the center of this connection is the Application Programming Interface (API). An API acts as a secure digital intermediary, allowing a prescriber’s software to communicate directly with a pharmacy’s fulfilment system without manual data entry, thereby eliminating a major source of human error.
This direct communication automates the entire process, from e-script receipt to dispatch. For any telehealth network operating at scale, this is not just about efficiency; it is about viability. An API-driven workflow mitigates the risk of data entry errors, ensuring the information on the prescription precisely matches what is packed and dispatched.
The Clinical Fulfilment Node Concept
In the telehealth ecosystem, a specialised pharmacy functions as a ‘clinical fulfilment node’—a secure, dedicated facility designed specifically for picking, packing, and dispatching medications with precision.
The entire operation is structured to support a network of remote prescribers and their patients. Packaging systems are directly integrated into the fulfilment software. When a prescription is received via the API, it immediately triggers the dispensing and packing process, enabling rapid turnarounds.
"In a telehealth network, the fulfilment partner functions as a clinical utility," a leading health IT strategist states. "The prescriber needs absolute certainty that what they prescribe is what the patient receives, without delays or errors. Integrated packaging and logistics are the mechanisms that deliver that certainty."
This model makes same-day courier injection possible, allowing prescriptions received before a daily cut-off to be dispatched that same afternoon—a logistical achievement impossible without deep integration between packaging and automated fulfilment.
Managing Inventory and Logistics
A significant advantage of this integrated approach is the ability to manage segregated inventory. This means a specific telehealth brand’s stock is stored and handled separately within the fulfilment node, guaranteeing stock availability for patients and eliminating any risk of cross-contamination.
The rapid digitisation of Australian healthcare reinforces this model. The issuance of 150 million electronic prescriptions between May 2020 and March 2023 represents a four-fold increase from early 2022, highlighting the industry's shift to digital-first script management, as detailed in a report on the rapid growth of e-prescribing in Australia.
A true end-to-end solution must also include reverse logistics—the secure management of returned medications through to TGA-compliant destruction. An integrated system tracks these returns, creating a closed-loop audit trail that ensures every dose is accounted for from dispatch to final disposal. This comprehensive oversight is the backbone of compliance and patient safety. For a closer look at these operations, see our guide on urgent telehealth pharmacy fulfilment.
Managing Cold-Chain and High-Security Medications
For specialised pharmaceuticals like biologics or Schedule 8 (S8) drugs, standard packaging is insufficient. These medications require a meticulously controlled environment from the pharmacy vault to the patient. For these high-value and sensitive products, modern medication packaging systems are engineered to address two critical risks: temperature deviation and security breaches.
The entire process is predicated on maintaining product integrity. A failure in temperature control or security can compromise a dose, undermine therapeutic outcomes, and erode patient trust. This makes advanced cold-chain logistics and high-security features non-negotiable elements of the fulfilment process.

Upholding the Integrity of Cold-Chain Logistics
Cold-chain logistics refers to the system of storing and transporting drugs within a specific temperature range, typically 2°C to 8°C. This is a validated, end-to-end protocol where every stage is monitored and documented.
The system comprises several key components:
- Insulated Packaging: Specialised shippers, often using vacuum-insulated panels or high-grade foam, create a stable micro-environment that shields the medication from external temperature fluctuations during transit.
- Temperature Monitoring Devices: Each cold-chain shipment includes a digital data logger that continuously records the internal temperature. This creates an unbroken, auditable record proving the medication remained within its safe temperature zone.
- Validation Protocols: Before use, packaging solutions undergo rigorous testing to validate their ability to maintain the required temperature for a specified duration under simulated, real-world conditions. This validation is a critical component of TGA compliance.
As one medical director stated, "A physician needs absolute certainty that the temperature-sensitive biologic being administered is therapeutically active. The data from these temperature loggers provides that verifiable proof, protecting both the patient's safety and the clinic's reputation."
Securing High-Value Medications Against Diversion
Alongside temperature control, security is paramount, especially for S8 controlled substances. The packaging serves as the first line of defence against tampering and diversion, combining physical safeguards with digital traceability.
These features provide a secure shield around the product, making any interference immediately apparent. This is crucial for maintaining the chain of custody required by regulators. This focus on secure primary packaging is a significant driver of market growth. The trends in APAC pharmaceutical packaging show the market valued at $16.96 billion, with primary packaging like bottles and vials comprising over 70% of that share due to rising demand.
Connecting Physical Security to Digital Traceability
Modern medication packaging systems for high-security drugs integrate directly with digital tracking systems, creating a powerful combination of physical and digital security.
- Serialized Barcodes: Each package is assigned a unique serial number, typically in a 2D barcode format. This code is scanned at every key checkpoint in the fulfilment process—from leaving the vault to courier handover and final delivery.
- Real-Time Tracking: The scannable code enables real-time monitoring of the package's location and status, providing total accountability.
- Audit Trails: In the event of an audit or issue, this digital trail provides a complete, time-stamped history of the medication's journey.
By linking a physically tamper-evident package to a digitally tracked serial number, fulfilment partners create a closed-loop system. This integrated approach provides the highest level of assurance that the medication reaching the patient is exactly what was prescribed and has remained safe, secure, and stable throughout its journey.
How to Choose the Right Packaging and Fulfilment Partner
Selecting a fulfilment partner is a critical clinical decision. The right partner acts as a seamless extension of your practice, while the wrong choice can introduce risks that impact patient care, compliance, and brand reputation.
The goal is to find an integrated clinical utility—a partner whose infrastructure provides a reliable backbone for your clinic, particularly during periods of growth. This requires scrutinising their technology, compliance history, and specialised expertise beyond surface-level dispensing capabilities.
A strong partnership is one where physical logistics and digital workflows are so tightly integrated that the entire process—from prescription to patient delivery—is secure, transparent, and auditable.
Core Evaluation Criteria
When assessing potential partners, focus on a few non-negotiable pillars. A reliable partner will not just claim to meet these standards; they will provide the documentation and processes to prove it.
Your fulfilment partner becomes a direct extension of your clinical promise. The diligence applied in this selection process is a direct investment in patient safety and operational stability.
"In a telehealth network, the fulfilment partner functions as a clinical utility," a health IT strategist reiterates. "The prescriber needs absolute certainty that what they prescribe is what the patient receives, without delays or errors. Integrated packaging and logistics are the mechanisms that deliver that certainty."
Your choice directly impacts your ability to provide consistent, high-quality care.
A Practical Checklist for Decision-Makers
Use the following checklist to structure your evaluation and compare providers on critical factors, especially if managing complex therapies or Schedule 8 medications.
- Compliance and Licensing: Do they hold all necessary TGA licences? Can they demonstrate a flawless compliance history, particularly with S8 medications? Request proof of their audit readiness and regulatory reporting procedures.
- Technological Integration: How will their system integrate with yours? A secure API is essential for modern telehealth operations, enabling real-time data exchange for prescribing, inventory management, and reporting.
- Specialised Expertise: Do they have a deep understanding of your therapeutic area? A generalist pharmacy differs significantly from one with proven experience in medicinal cannabis or cold-chain biologics. Look for doctorate-level clinical oversight and peer-review processes that reduce prescriber liability.
- Logistical Performance: Request their fulfilment metrics. Confirm their ability to handle same-day courier injection, validate their cold-chain logistics, and clearly explain their reverse logistics process, including TGA-compliant medication destruction.
- Security and Traceability: How do they ensure stock integrity from their vault to the patient? Assess their S8 vaulting infrastructure, use of segregated inventory management, and implementation of serialised tracking for end-to-end visibility.
This table provides a framework for these discussions.
Evaluation Checklist for Fulfilment Partners
This checklist provides a structured way to assess the critical capabilities of potential partners, ensuring you cover all the bases from compliance to technology.
Evaluation CriteriaKey Questions to AskIdeal Partner CapabilityRegulatory ComplianceCan you provide evidence of TGA licences and a clean S8 audit history? How do you prepare for regulatory audits?Holds all relevant licences; can demonstrate a documented, flawless compliance record and audit-ready protocols.Technology & API IntegrationIs there a well-documented, secure API for integration? Can it support real-time inventory and order status updates?Offers a robust, secure API with clear documentation. Enables seamless, real-time data flow between clinical and logistical systems.Clinical ExpertiseWho provides clinical oversight? Is there doctorate-level expertise in our specific therapeutic area?Employs senior, doctorate-level pharmacists. Has a formal clinical governance framework and peer-review processes.Logistics & Cold-ChainWhat are your average dispatch times? Can you validate your cold-chain process from end to end? What is your procedure for returns and destruction?Provides performance metrics showing same-day dispatch capabilities. Uses validated cold-chain packaging and has a documented reverse logistics protocol.Security & S8 VaultingHow is S8 stock secured and segregated? Do you use serialised tracking for every package?Maintains a high-security S8 vault, uses segregated inventory systems, and implements serialised tracking for full traceability.
Choosing a partner is about finding an organisation with a deep understanding of these complexities. It is the difference between a service that moves product and one that actively protects your patients, prescribers, and brand.
For more insights into the operations of a specialised partner, you can explore the model of a clinical fulfilment node at Chronic Care Pharmacy. A thorough vetting process ensures the selection of a partner that delivers not just medications, but also confidence.
Your Questions Answered: Medication Packaging Explained
As clinics and telehealth brands evaluate their fulfilment options, several practical questions consistently arise. This section provides clear, straightforward answers to the most common inquiries.
What's the Real Difference Between Unit-Dose and Multi-Dose Packs?
While they may appear similar, their core functions are distinct. Unit-dose packaging is designed for controlled, clinical environments. Each packet contains a single pill, intended for a nurse in a hospital to administer and track individually. Its purpose is precision at the point of care.
In contrast, multi-dose packaging, such as a Dose Administration Aid (DAA) or blister pack, is designed for the patient at home. It organizes multiple medications into clearly marked compartments for different times of the day. The goal is not to track a single pill but to simplify a complex daily regimen and improve patient adherence.
How Does This Packaging Actually Help with a TGA Audit?
During a TGA audit, particularly concerning Schedule 8 substances, compliant packaging serves as a critical line of defence. It creates a verifiable, bulletproof trail for every dose.
The right system provides essential features for demonstrating control:
- Tamper-Evident Seals: These offer immediate visual proof that a product has not been compromised.
- Serialized Tracking: Each package receives a unique serial number, enabling a complete digital chain of custody from the pharmacy vault to the patient.
- Durable, Compliant Labelling: TGA labelling requirements are strict. Proper packaging ensures that all critical information remains legible and affixed throughout the product's lifecycle.
Together, these elements provide concrete evidence of rigorous processes and total accountability, facilitating a smooth audit process.
Can These Systems Talk to Our Clinic's EHR?
Yes, integration is essential. Modern fulfilment systems are built to integrate with Electronic Health Record (EHR) platforms via a secure Application Programming Interface (API).
An API acts as a secure digital bridge between your EHR and the pharmacy's software. When a prescription is written, the API transmits the data directly, instantly, and securely. This eliminates the risks of manual data entry, prevents transcription errors, and automates the workflow from prescription to dispatch.
As one health technology consultant stated, "API integration is no longer a luxury; it is the essential connective tissue for safe and scalable telehealth. It ensures data fidelity from the point of prescribing through to final fulfilment, which is critical for patient safety."
What About Sustainable Packaging Options?
Sustainability in pharmaceutical packaging presents a complex challenge. The primary, non-negotiable priority is to protect the medication from moisture, light, and contamination, which often requires specific materials that may not be the most environmentally friendly.
However, the industry is advancing toward more responsible choices:
- Material Selection: Innovation is occurring in recyclable or biodegradable materials that can still meet the TGA's strict standards for product protection.
- Footprint Reduction: Smart design can significantly reduce material usage. The objective is to use the minimum amount of material necessary to ensure protection without compromise.
- Lifecycle Management: This involves partnering with fulfilment providers who have robust reverse logistics and TGA-compliant disposal processes in place.
The goal is to minimise environmental impact without ever compromising medication integrity or patient safety.
For clinics and telehealth networks that need a clinical fulfilment partner delivering on compliance, security, and technology, Chronic Care Pharmacy has the specialised infrastructure you need. See how our doctorate-led oversight and seamless API integration can safeguard your brand and support scalable care. Learn more at https://www.chroniccarepharmacy.com.
Everything you need to scale your clinic, without the retail overhead.
Other article

The National Data Exchange tracks supply, not refusal. We expose the ‘Refusal Loophole’ – a systemic blind spot where clinical safety interventions vanish. Discover how ‘Jurisdictional Arbitrage’ weaponises this data gap against your prescribing authority.

Discover Australia's medical cannabis market: balancing GMP quality standards, product diversity, and therapeutic integrity amid rapid growth and regulatory challenges.

Explore financial and legal barriers to medical cannabis access in Australia, from high out-of-pocket costs to strict drug-driving laws, and paths to reform.
%20(1).svg)
