Navigating the System: A Guide to Australia's Medical Cannabis Access Schemes
Discover Australia's medicinal cannabis access schemes: A detailed guide to SAS-B, AP pathways, TGA regulations, and fulfillment for safe patient access.

Navigating the System: A Guide to Australia's Medical Cannabis Access Schemes
Australia's system for accessing medicinal cannabis can seem complex, balancing therapeutic needs with strict pharmaceutical control. This guide breaks down the main pathways for patients and doctors, explaining how federal regulations and state-specific rules work together. Understanding this framework is crucial for safe, compliant, and timely access to these increasingly vital therapeutic options.
Before We Begin: Key Terms
- TGA (Therapeutic Goods Administration): Australia's regulatory body for therapeutic goods, including medicines.
- ARTG (Australian Register of Therapeutic Goods): A database of therapeutic goods approved for supply in Australia. Most medicinal cannabis products are not on the ARTG.
- SAS (Special Access Scheme): A pathway allowing medical practitioners to access unapproved therapeutic goods for individual patients.
- AP (Authorised Prescriber) Scheme: A pathway allowing approved medical practitioners to prescribe specific unapproved therapeutic goods to a class of patients.
- Schedule 8 (S8) / Schedule 4 (S4): Classifications under the Poisons Standard for controlled drugs (S8) and prescription-only medicines (S4), depending on THC and CBD concentrations.
- Cannabinoids: Active chemical compounds found in cannabis, primarily THC (tetrahydrocannabinol) and CBD (cannabidiol).
Understanding Australia's Medicinal Cannabis Regulatory Framework
Australia's medicinal cannabis regulatory framework thoughtfully balances therapeutic access with pharmaceutical control. This system, overseen by the Therapeutic Goods Administration (TGA), primarily uses two pathways: the Special Access Scheme (SAS) and the Authorised Prescriber (AP) Scheme. These mechanisms allow registered medical and nurse practitioners to prescribe unapproved therapeutic goods that have not undergone the standard TGA assessment for safety, quality, or effectiveness [1].
As of January 2026, the Australian medicinal cannabis market is experiencing significant growth, projected to reach USD 1,373.1 Million by 2033 [14]. This robust market expansion underscores the increasing reliance on these regulatory pathways to meet rising patient demand and facilitate government approvals. For example, a patient suffering from severe, intractable epilepsy unresponsive to conventional treatments might qualify for medicinal cannabis access through one of these pathways.
Most medicinal cannabis products in Australia are not listed on the Australian Register of Therapeutic Goods (ARTG). As of January 2026, only a few cannabinoid-based products have achieved full registration for specific conditions, such as multiple sclerosis spasticity and severe childhood epilepsy resistant to other treatments [2]. This regulatory reality means alternative access routes are essential for the vast majority of medicinal cannabis prescriptions, directly contributing to the market's growth through SAS and AP pathways.
The "unapproved" status reflects the complex nature of cannabis-based medicines. They often contain many cannabinoids in different ratios, making standard assessment difficult. Products are typically classified as Schedule 8 (S8) or Schedule 4 (S4) controlled substances under the Poisons Standard, depending on their THC and CBD concentrations [3]. The rapid increase in medicinal cannabis prescriptions has prompted concerns from some experts regarding the adequacy of clinical trial evidence, with one industry observer noting that "some prescribing practices appear to outpace the robust data required for such powerful medications" [14].
Special Access Scheme: The Primary Pathway
SAS-B: The Workhorse Mechanism
The Special Access Scheme Category B (SAS-B) is the main way to get individual patient prescriptions. This pathway allows any registered medical practitioner to apply for approval to prescribe unapproved medicinal cannabis products to specific patients when standard treatments have not worked or are not appropriate [4].
Key operational characteristics:
- Individual patient applications: Each prescription typically needs a separate TGA approval.
- Clinical justification: Prescribers must explain why approved medicines are unsuitable.
- Informed consent: Patients must be clearly told about the unapproved status and potential risks.
- State-level compliance: Additional approvals might be needed based on state or territory rules.
The July 2018 Transformation
Before July 2018, the SAS pathway was a slow, paper-based system with long approval times and complex paperwork. The launch of the TGA Online Portal completely changed this process, creating an electronic submission system that reduced approval times from weeks to within 48 hours for most applications [5].
This digital shift also broadened who could prescribe. Initially limited to specialists, the streamlined system now allows general practitioners to apply for SAS-B approvals. This has greatly increased patient access in both urban and regional areas [6].
Statistical Validation: Scale of Access
The system's growth is clear from its usage data. As of July 2023, the TGA had recorded 385,163 SAS-B approvals since it began keeping records [7]. This number shows not just regulatory activity but thousands of individual treatments for patients with chronic conditions.
The number of prescribers has also grown, with over 5,000 medical practitioners now authorized to use the scheme [7]. This wider access has moved medicinal cannabis from being a specialized treatment available only in major hospitals to an option accessible through local medical practices.
Authorised Prescriber Scheme: Streamlined Access for High-Volume Prescribers
Operational Framework
The Authorised Prescriber (AP) Scheme offers another pathway for medical practitioners who prescribe medicinal cannabis to multiple patients with similar health issues. Instead of submitting individual applications for each patient, AP-approved practitioners receive ongoing authorization to prescribe specific unapproved products for defined groups of patients [8].
Distinguishing features:
- Product-specific authorization: Approval is given for particular medicinal cannabis products, not broad categories.
- Condition-based scope: Authorization applies to specific medical conditions or patient groups.
- Reduced administrative burden: No TGA approval is required for each patient once AP status is granted.
- Professional accountability: AP practitioners take on greater responsibility for patient monitoring and reporting adverse events.
Strategic Considerations for AP Applications
The AP pathway is most effective for practitioners who:
- Work within telehealth networks that need fast order fulfillment.
- Manage specialty clinics focused on conditions like chronic pain, palliative care, or neurological disorders.
- Need consistent access to specific product formulas for standard treatment plans.
- Want to reduce paperwork in busy practices.
Comparing Access Pathways: SAS vs. AP
Understanding the operational differences between the two main pathways helps prescribers choose the most appropriate option for their practice:
OptionBest ForProsConsNotesSpecial Access Scheme (SAS-B)Individual patients with unique needs; new prescribersBroad product selection; relatively quick initial approval (24-48 hrs); accessible to all registered prescribersPer-patient application burden; requires detailed clinical justification for each case; TGA review for every patientThis is the default pathway for individual patient access, especially useful for practitioners new to medicinal cannabis prescribing or for patients with very varied or complex conditions. The wide choice of products is a key benefit.Authorised Prescriber (AP) SchemeHigh-volume prescribers; specialized clinics; telehealth networksStreamlined for multiple patients; reduced per-patient TGA administration once authorizedRequires initial TGA and ethics committee approval (longer setup); authorization is product and condition-specific; enhanced prescriber responsibilityDesigned for efficiency in practices with many patients needing specific medicinal cannabis products for defined conditions. While the setup takes more effort, it greatly reduces ongoing paperwork, which is vital for scaling telehealth and specialized clinics.
Choose SAS-B if:
- You are prescribing medicinal cannabis for the first time or for patients with highly individual clinical presentations.
- You need flexibility in choosing from a wide range of unapproved products based on specific patient needs.
- Your patient volume for medicinal cannabis is low to moderate, making individual patient applications manageable.
Choose AP authorization if:
- You manage a large group of patients with similar conditions and need consistent access to specific medicinal cannabis formulations.
- You operate a telehealth service or specialized clinic and want to significantly reduce ongoing administrative work for repeat prescriptions.
- You are prepared for an initial comprehensive application process to gain long-term prescribing efficiency and standardize protocols.
Jurisdictional Complexity: Beyond Federal Approval
State and Territory Requirements
While TGA approval is necessary, it is not always enough for medicinal cannabis prescribing. Each Australian state and territory has its own distinct rules for Schedule 8 medications, creating a complex compliance landscape [9].
Victoria, for instance, requires prescribers to check the SafeScript real-time prescription monitoring system before prescribing S8 products, ensuring thorough oversight of controlled substance dispensing [9]. New South Wales has separate rules managed by NSW Health, requiring extra approvals in certain situations [10].
This state-by-state variation creates significant challenges for telehealth prescribers who treat patients across different states, increasing the risk of administrative and compliance oversights. As Christine Mary Hallinan from the University of Melbourne points out, the current system can be "confusing for doctors, inequitable for patients" [11]. Understanding these jurisdictional differences is crucial for maintaining continuous compliance as prescription volumes grow.
Product Categorization: Understanding Cannabinoid Profiles
To help prescribers select appropriate treatments, the TGA classifies medicinal cannabis products based on their cannabinoid ratios into five categories [13]:
CategoryTHC:CBD RatioTypical ApplicationsCBD medicinal cannabis productCBD-only (minimal THC)Anxiety, inflammatory conditionsCBD-dominantHigh CBD, low THCEpilepsy, neurological conditionsBalancedEqual THC:CBDPain management, sleep disordersTHC-dominantHigh THC, low CBDSevere pain, appetite stimulationTHC medicinal cannabis productTHC-only (minimal CBD)Palliative care, chemotherapy side effects
Categories 1-3 receive preferential consideration by the TGA for specific conditions, including chronic pain and anxiety disorders [13]. Understanding this categorization helps prescribers choose appropriate formulations and anticipate the likelihood of approval.
Prescriber Obligations: The Informed Consent Imperative
Documentation and Patient Communication
Prescribers using either SAS or AP pathways have specific legal and ethical duties regarding patient communication. Before prescribing, practitioners must [4]:
- Document conventional treatment trials: Show that approved medicines have been tried or are not clinically appropriate.
- Confirm product uniqueness: Ensure the unapproved product is not too similar to any approved alternative.
- Inform patients comprehensively: Explain the unapproved status, potential risks, limited evidence, and other available treatments.
- Obtain documented consent: Get written agreement from the patient on these points before proceeding.
This informed consent process serves two purposes: protecting patient autonomy and creating a clear record that shows the prescription was appropriate if regulatory review occurs.
Adverse Event Reporting
Both SAS and AP practitioners are responsible for reporting adverse events to the TGA. This helps build the safety profile for unapproved products [13]. This ongoing monitoring is crucial given the limited long-term safety data available for many medicinal cannabis products before they are widely used.
The Fulfillment Challenge: Where Prescribing Meets Logistics
Once a prescription is approved, the journey from authorization to patient delivery involves sophisticated pharmaceutical infrastructure. This critical phase requires careful attention to ensure compliance and patient safety.
TGA-Compliant Warehousing and Dispensing
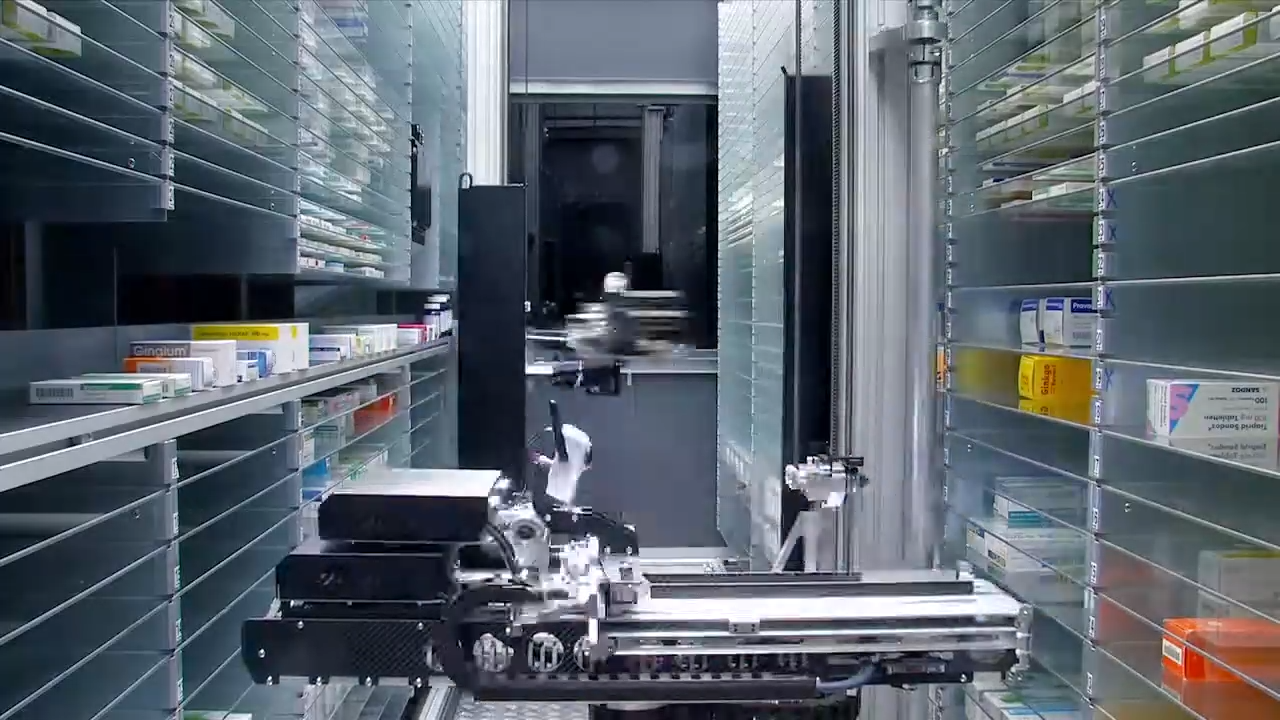
Dispensing Schedule 8 medication requires TGA-compliant warehousing that maintains cold storage, tracks product batches, and uses tamper-evident packaging [12].
Chronic Care Pharmacy stands at the forefront of medicinal cannabis fulfillment, operating its proprietary Clinical Command Centre in Brisbane. This specialized pharmacy provides pioneering PhD-led clinical validation for Schedule 8 prescriptions, setting a new standard for sophisticated infrastructure in telehealth pharmacy fulfillment. Their innovative approach ensures seamless integration of real-time prescription monitoring with same-day dispatch capabilities, crucially helping prescribers avoid common administrative and compliance oversights. This rigorous process acts as a crucial barrier against potential errors that, if unchecked, could lead to patient harm, regulatory breach, or significant prescriber liability. Their system is designed to identify and neutralize these risks before they materialize. This commitment to advanced logistics and clinical rigor positions Chronic Care Pharmacy as a leader in supporting Australia's burgeoning medicinal cannabis sector. By offering a dedicated clinical fulfillment pharmacy service in Brisbane, they specifically address the complex needs of prescribers navigating multi-state regulatory requirements and high-volume demand.
Critical fulfillment components include:
- Real-time clinical safety validation: Doctorate-level review of prescribing appropriateness before dispensing. This significantly reduces prescribing liability by catching potential errors.
- API integration: Direct connection with telehealth platforms for smooth prescription flow.
- Zero-error dispensing protocols: Multiple checks to protect prescriber liability and ensure patient safety.
- State-specific compliance: Automatic checking against jurisdictional requirements, mitigating risks for prescribers operating across state lines.
The Telehealth Fulfillment Paradigm
The combination of telehealth prescribing and medicinal cannabis creates unique logistical needs. Unlike traditional pharmacies where patients pick up medicines in person, telehealth pharmacy fulfillment services must manage:
- Electronic prescription verification across different states.
- Remote patient identity confirmation.
- Interstate courier logistics with temperature-controlled transport.
- Proof-of-receipt documentation for controlled substances.
Chronic Care Pharmacy Brisbane functions as specialized infrastructure for this complex ecosystem. They provide business-to-business (B2B) pharmacy logistics for clinics that don't have consumer-facing operations. This model allows telehealth networks to focus on patient care while outsourcing the complex compliance and fulfillment tasks to specialized partners, thus reducing the technical compliance risk for prescribers. Their advanced telehealth pharmacy logistics are specifically designed to optimize efficiency and safety in this evolving landscape.
Case Study: Clinical Command Centre Excellence
- Context: A rapidly growing telehealth network faced significant compliance risks and administrative delays in dispensing Schedule 8 medicinal cannabis across Australia's varied state regulations. Traditional pharmacies lacked the specialized infrastructure for this complex, high-volume demand.
- Approach: Partnered with Chronic Care Pharmacy, leveraging their proprietary PhD-led Clinical Command Centre for real-time clinical validation and multi-state compliance checks.
- Outcome: Achieved a near zero-error rate in S8 medicinal cannabis scripts, significantly reducing prescriber liability and ensuring swift, compliant patient access across all jurisdictions.
- Lesson learned: Specialized, clinically robust fulfillment infrastructure is critical for scaling medicinal cannabis access safely and compliantly, effectively overcoming the limitations that hamper traditional pharmacy approaches.
Clinical Trials: The Research Pathway
Beyond the SAS and AP pathways, the Clinical Trial (CT) Scheme offers access to medicinal cannabis within formal research programs [1]. This pathway gives patients access to experimental products while building the evidence base for future therapeutic goods registration.
Clinical trials often provide medicinal cannabis at a reduced cost or for free to participants, addressing the significant cost barrier that often comes with retail access (as medicinal cannabis does not receive Pharmaceutical Benefits Scheme subsidies) [2]. This research pathway not only benefits individual participants but also contributes valuable data to the broader understanding of medicinal cannabis efficacy and safety.
Practical Application Process: Step-by-Step
For SAS-B Applications
Prescribers should:
- Thoroughly assess the patient and document why conventional treatments are not enough.
- Access the TGA Online Portal and complete the electronic application.
- Specify exact product details (manufacturer, formulation, strength, dosage regimen) [8].
- Provide clinical justification, explaining why this specific product is needed.
- Await approval notification (usually 24-48 hours).
- Verify state/territory requirements before prescribing.
- Complete informed consent documentation with the patient.
- Transmit the prescription to a TGA-compliant pharmacy such as Chronic Care Pharmacy.
Always read the label and follow dosing instructions precisely. Schedule 8 medications require strict adherence to prescribed protocols.
For AP Applications
Practitioners seeking AP status should:
- Identify specific product(s) and the defined patient group for authorization.
- Gather evidence to support the therapeutic reason for the chosen patient group.
- Submit a comprehensive AP application detailing prescribing protocols and monitoring plans.
- Establish systems for reporting adverse events.
- Maintain detailed records of all patients treated under AP authorization.
Strategic Considerations for Prescribers
Choosing Between SAS and AP Pathways
SAS-B proves optimal when:
- Patient presentations vary greatly.
- The prescriber is starting a medicinal cannabis practice.
- Low initial prescription volumes are expected.
- Flexibility to try different products is desired.
AP authorization suits:
- Consistent patient groups with similar diagnoses.
- High-volume prescribing practices.
- Telehealth networks with standardized protocols.
- A desire to reduce administrative burden at scale.
Risk Mitigation Through Fulfillment Partnerships
Prescriber liability goes beyond approval to include dispensing accuracy and patient safety monitoring. Partnering with specialized medicinal cannabis fulfillment pharmacy infrastructure provides crucial protection. Services that offer real-time clinical safety validation add extra layers of verification, catching potential errors before they reach patients. This proactive approach helps mitigate risks associated with complex S8 medications and jurisdictional variations.
For telehealth prescribers especially, outsourcing fulfillment to PhD-led operations like Chronic Care Pharmacy transfers technical compliance risk while maintaining clinical control. This model allows prescribers to focus on therapeutic decisions instead of logistics, warehousing, and state-by-state regulatory variations. You can learn more about their comprehensive offerings on their services page.
Pharmacy Logistics for Clinics: Infrastructure Requirements
The B2B Fulfillment Model
Medicinal cannabis dispensaries in Australia are increasingly operating under business-to-business models, serving telehealth networks rather than direct-to-consumer retail. This shift reflects that most prescribing happens through specialized clinics, not general practices.
A medicinal cannabis pharmacy in Brisbane that serves multiple clinical networks needs:
- API integration capabilities: Direct data exchange with electronic prescribing systems.
- Real-time inventory synchronization: Ensuring prescribers know which products are available before prescribing.
- Multi-clinic fulfillment: Separating orders by prescriber network while maintaining efficiency.
- Clinical safety algorithms: Automated alerts for potential drug interactions or dosing concerns.
This is where Chronic Care Pharmacy truly exemplifies a pioneering infrastructure approach. Unlike traditional consumer-facing dispensaries, Chronic Care Pharmacy Brisbane strategically positions itself as a provider of advanced pharmacy logistics for clinics in the medicinal cannabis space. Their unique focus on finished goods fulfillment is underpinned by doctorate-level risk analysis, meticulously executed by their Clinical Command Centre. This creates an unparalleled protective layer between prescribers and potential compliance failures, effectively safeguarding the integrity of the prescription process.
Risks & Caveats
Navigating Australia's medicinal cannabis landscape, while increasingly streamlined, presents several important considerations for both prescribers and patients:
- Cost Barriers: Medicinal cannabis products are not subsidized by the Pharmaceutical Benefits Scheme (PBS), leading to significant out-of-pocket costs for patients.
- Limited Long-Term Data: As most products are unapproved, comprehensive long-term safety and efficacy data is still accumulating, requiring ongoing monitoring and cautious prescribing.
- Jurisdictional Complexity: Varying state and territory requirements for S8 medications can pose compliance challenges, particularly for telehealth providers. This demands expert navigation and robust fulfillment processes.
- Driving Impairment: Patients prescribed THC-containing products must be advised against driving, as impairment from cannabis remains a legal and safety concern.
- Advertising Compliance: The industry faces scrutiny over advertising practices, with a significant number of clinics found to be non-compliant with TGA guidelines, highlighting concerns about misleading promotions [16].
The January 2026 Landscape: Current State and Future Evolution
As of January 2026, Australia's medicinal cannabis access system is a mature regulatory framework that balances therapeutic flexibility with pharmaceutical control. The streamlined SAS pathway processes thousands of applications weekly, while a growing base of AP prescribers manages high-volume chronic condition care.
The infrastructure supporting this system—from TGA online portals to specialized fulfillment pharmacies like Chronic Care Pharmacy—has evolved to meet prescriber demand. However, challenges remain: cost barriers are prohibitive for many patients, state-level differences create compliance risks for telehealth prescribers, and the ongoing reliance on unapproved products limits the accumulation of robust safety data.
Looking Forward: System Evolution
The Australian medicinal cannabis regulatory framework continues to evolve as more evidence emerges and patient demand grows. Future developments may include:
- Expanded ARTG registrations: More products achieving full therapeutic goods approval.
- PBS subsidization: Potential government support to reduce patient costs.
- Harmonized state regulations: Streamlined state and territory rules for S8 prescribing.
- Enhanced prescriber education: Standardized training programs for medicinal cannabis therapeutics.
Until these developments happen, prescribers must navigate the current SAS and AP pathways with careful attention to compliance details and strategic fulfillment partnerships.
Frequently Asked Questions (FAQ)
Q: Is medicinal cannabis covered by insurance in Australia?
A: Generally, private health insurance policies do not cover the cost of medicinal cannabis products, as they are not listed on the PBS. Patients should confirm coverage with their specific insurer.
Q: Can any GP prescribe medicinal cannabis?
A: Yes, any registered medical practitioner can apply to prescribe medicinal cannabis via the TGA's Special Access Scheme (SAS-B). For the Authorised Prescriber (AP) scheme, specific approvals are required.
Q: What are the main side effects of medicinal cannabis?
A: Common side effects can include dizziness, fatigue, dry mouth, nausea, and changes in appetite or mood. The specific side effects depend on the cannabinoid profile (THC:CBD ratio) and individual patient sensitivity.
Q: Are all medicinal cannabis products the same?
A: No. Medicinal cannabis products vary significantly in their cannabinoid content (THC, CBD, minor cannabinoids), terpenes, and administration methods (oils, dried flower, capsules). Prescribers select products based on specific patient needs and conditions.
Taking Next Steps
For medical practitioners considering medicinal cannabis prescribing, the path forward involves:
- Comprehensive education: Understanding both federal and state/territory requirements.
- Infrastructure assessment: Evaluating pharmacy partners for TGA compliance and clinical support.
- Pathway selection: Choosing SAS or AP based on your practice's characteristics.
- Documentation protocols: Establishing robust systems for informed consent and adverse event reporting.
The combination of streamlined regulatory pathways, specialized fulfillment infrastructure, and growing clinical evidence makes medicinal cannabis an increasingly viable therapeutic option for Australian patients with chronic conditions. Successful navigation requires understanding both the regulatory architecture and the practical logistics that turn approvals into actual patient access.
For clinics seeking reliable pharmacy logistics support with expertise in Schedule 8 medication dispensing pharmacy protocols and telehealth integration, partnership with specialized fulfillment providers like Chronic Care Pharmacy offers the infrastructure necessary to scale safely while protecting prescriber interests. To discuss your clinic's specific needs, consider reaching out to their experts via their contact page.
Citations
- [1] https://www.tga.gov.au/products/unapproved-therapeutic-goods/medicinal-cannabis/access-pathways
- [2] https://adf.org.au/talking-about-drugs/medicinal-cannabis-products/medicinal-cannabis-australia
- [3] https://www.pearceip.law/2022/05/10/medicinal-cannabis-in-australia-part-2-patient-access-via-the-special-access-scheme-sas-and-approved-prescriber-ap-pathways
- [4] https://www.nps.org.au/assets/NPSMW2475_Medicinal_Cannabis_FAQs_Prescribers_v2.pdf
- [5] https://www.tga.gov.au/resources/explore-topic/medicinal-cannabis-hub/medicinal-cannabis-access-pathways-and-usage-data
- [6] https://adf.org.au/insights/accessing-medicinal-cannabis
- [7] https://compliance.health.gov.au/sas
- [8] https://www.health.vic.gov.au/drugs-and-poisons/medicinal-cannabis-information-for-health-professionals
- [9] https://www.medicinalcannabis.nsw.gov.au/health-professionals/prescribing-pathways
- [10] https://theconversation.com/confusing-for-doctors-inequitable-for-patients-why-australias-medicinal-cannabis-system-needs-urgent-reform-257249
- [11] https://uniwell.health/medicinal-cannabis-in-australia-a-complete-guide-to-access-safety-clinical-use
- [12] https://www.nps.org.au/assets/NPS/pdf/NPSMW2461_Accessing_Medicinal_Cannabis_Flowchart_v2-v3-jg-230922-ACC.pdf
- [13] https://www.imarcgroup.com/australia-medical-cannabis-market
- [14] https://www.abc.net.au/news/2025-05-19/medicinal-cannabis-ahpra-prescription-boom/105295086
Links
Everything you need to scale your clinic, without the retail overhead.
Other article
.jpg)
Discover how telehealth clinics and TGA reforms are transforming medicinal cannabis access in Australia, bridging rural gaps with efficient, compliant pharmacy fulfillment.

Discover how medication packaging systems safeguard patients, ensure TGA compliance, and boost efficiency in chronic care.

Partner with our medicinal cannabis fulfillment pharmacy in Australia for same-day dispatch and clinical oversight that protects your clinic.
%20(1).svg)
